Abstract
Background:
Primary central nervous system lymphoma (PCNSL) is a rare type of extranodal non-Hodgkin lymphoma (NHL). Most cases occur in the immunocompetent population. Despite advances in therapy, the prognosis of PCNSL remains poor, with 5-year overall survival (OS) rates at 30-50% (Ferreri 2011). The standard of care treatment regimen includes high-dose methotrexate (HD-MTX), often in combination with other multiagent CNS penetrating chemotherapies. Although the addition of rituximab in systemic aggressive B-cell NHLs has shown improvement in OS, the benefit of this agent in PCNSL has not been confirmed in a prospective dataset (Bromberg 2017). The majority of PCNSLs are classified as diffuse large B-cell lymphomas (DLBCL), and the outcomes based on treatment and cell of origin (germinal center B-cell (GCB) vs. non-GCB subtype) have yet to be fully elucidated. In this review, we report our findings from a series of PCNSL patients based on treatment regimen and cell of origin.
Methods:
This is a retrospective study of patients with PCNSL diagnosed and treated at the University of Colorado Hospital from 1995 to 2017. Chart abstraction included demographics, DLBCL subtype by Hans algorithm (Hans 2004), treatment details, and outcomes. OS was examined using Kaplan Meier methods, while bootstrap resampling and a z-test was used to compare median survival times across strata. Fisher's exact test was used to determine whether treatment responses were different between those that received treatment withHD-MTX and rituximab versus HD-MTX without rituximab. A significance level of 0.05 was used for all tests. When assessing differences by subtype, treatment type, relapse, and treatment response the data was subset to include only patients without a prior HIV diagnosis.
Results:
71 patients with PCNSL were identified. 13 patients were HIV positive (the HIV status of 1 patient was unknown). Of the remaining 57 immunocompetent patients, 39 patients were treated with HD-MTX, of which 29 patients received rituximab in combination therapy. The most commonly received regimen was rituximab with methotrexate, procarbazine, and vincristine (R-MPV) (43.6%, 17 of 39). Median age in the HD-MTX subgroup was 65 years, with 83% of patients with KPS>70 at diagnosis. EBV positivity was noted in only 4 of 39 samples (10.2%), while 25 of 39 (64.1%) tested negative; the remaining patients' data was not available.
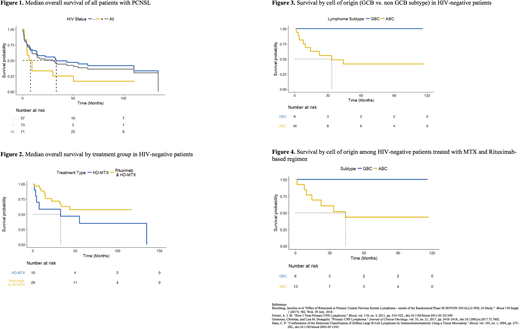
Median OS was 29.9 months for all 71 patients (Figure 1). There was no significant difference in median OS between those with and without HIV (p=0.127).
22 of 29 (75.9%) patients in the HD-MTX with rituximab group achieved a complete response (CR) while no patients (0%; 0 of 10) in the HD-MTX without rituximab group achieved a CR. The number of patients with a CR was significantly higher for those that received HD-MTX with rituximab compared to those that received HD-MTX without rituximab (p<0.001).
Median OS for HIV negative patients treated with HD-MTX without rituximab (N = 10) was 32.6 months whereas median OS for HIV negative patients treated with HD-MTX with rituximab (N = 29) has not been reached at a median follow up of 33.4 months (range 1.5 - 116.9 months) (Figure 2)
Subtyping was available for 23 patients without HIV treated with HD-MTX with or without additional agents. 6 patients had GCB subtype and 16 had non-GCB subtype. Median OS for GCB subtype has not yet been reached, while median OS for non-GCB subtype was 33.4 months (Figure 3). When analyzing data for patients who received HD-MTX and rituximab, median OS for GCB has not been reached, while median OS for non-GCB was 43.8 months (Figure 4).
Conclusions:
Our review of patients with PCNSL suggests that the addition of rituximab to a high dose MTX-based regimen improves survival although numbers are too small to determine any overarching statistical conclusion. CR rates were significantly higher in patients receiving rituximab in addition to HD-MTX compared to HD-MTX without rituximab. Patients with non-GCB subtype treated with HD-MTX and rituximab had increased median OS compared to median OS of non-GCB patients treated with HD-MTX without rituximab. However, in line with previous data we demonstrated worse outcomes associated with non-GCB subtype compared to GCB subtype. Future research is needed to focus on the characteristics of this subtype of PCNSL to improve outcomes with novel therapeutics.
Kamdar:Genentech: Consultancy; Seattle Genetics: Speakers Bureau. Haverkos:Viracta Therapeutics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.